X chromosome inactivation is a fascinating biological process that allows female mammals to balance gene expression from their two X chromosomes. Unlike males, who possess a single X chromosome, females must silence one copy to prevent an overabundance of X-linked gene products, which can lead to disorders. This intricate mechanism is pivotal in understanding various X-linked diseases, including Fragile X Syndrome and Rett Syndrome. Recent research highlights the potential for novel chromosomal therapies aimed at reactivating silenced genes, providing hope for those affected by genetic disorders caused by mutations on the X chromosome. With advancements in this field, the promise for effective treatments is becoming a reality for many individuals living with these challenging conditions.
The phenomenon known as X chromosome silencing represents a crucial regulatory mechanism in genetics, particularly in females where two X chromosomes are present. This process, often referred to as X-inactivation, ensures that gene dosage remains balanced in organisms. It plays a significant role in a range of genetic disorders, particularly X-linked diseases that can impact both genders. Emerging chromosomal therapies focus on addressing these disorders by potentially reactivating genes that have been inactivated. As we deep dive into the complexities and functions of this biological system, new discoveries are promising for effective intervention strategies for conditions such as Fragile X and Rett syndromes.
Understanding X Chromosome Inactivation
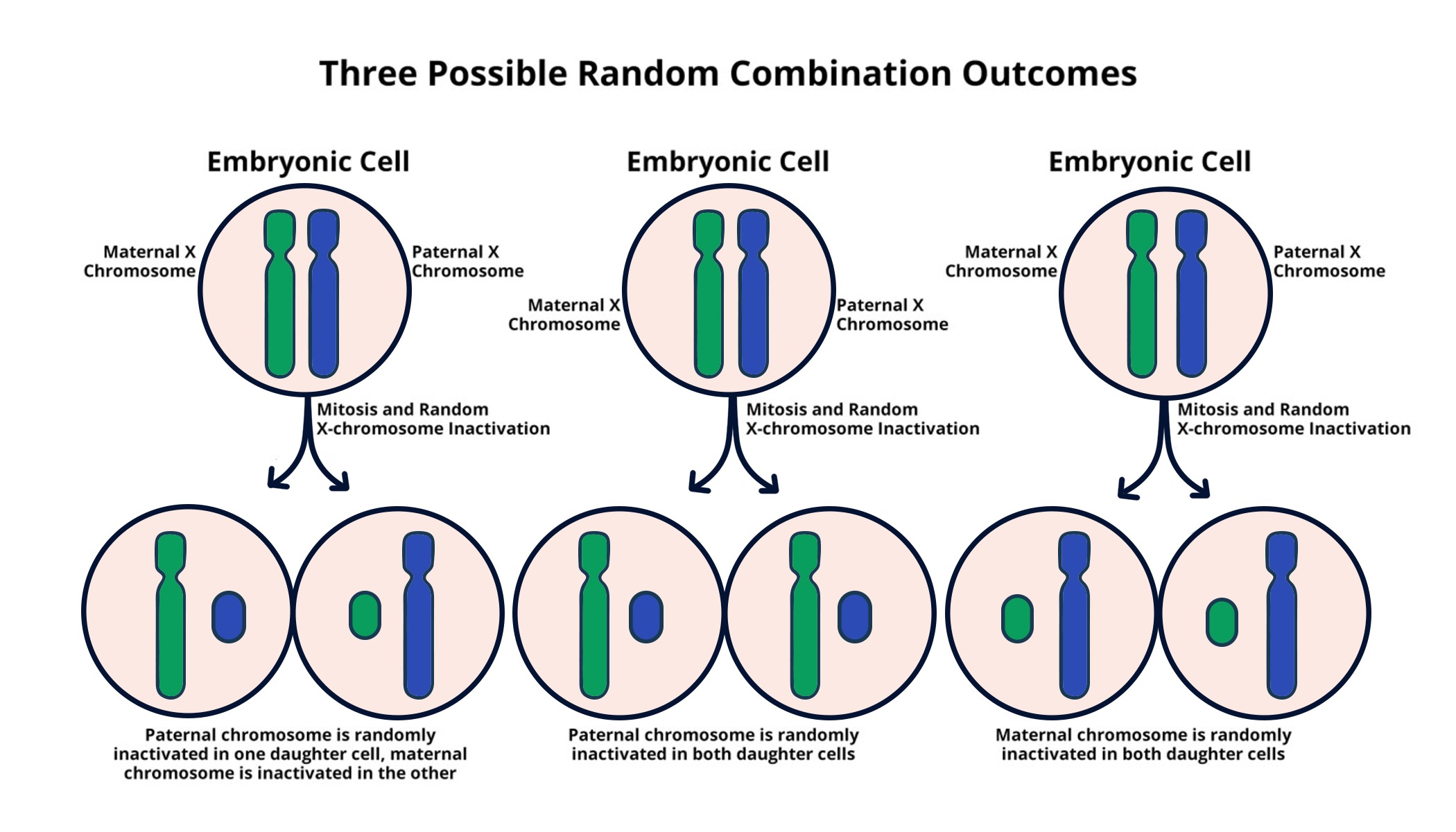
X chromosome inactivation (XCI) is a critical biological process that happens in females to balance gene dosage between sexes. In essence, one of the two X chromosomes in every female cell is randomly silenced to ensure that the gene products from the X chromosome are equivalent to those in males, who possess only one X chromosome. This intricate process is not simply a cellular response; it plays a crucial role in the onset of various X-linked diseases, including Fragile X syndrome and Rett syndrome. Researchers like Jeannie Lee have dedicated years to delving into the mechanisms underlying this fascinating phenomenon, aiming to uncover therapeutic pathways that could lead towards curing several genetic disorders linked to faulty genes on the X chromosome.
The process begins with the production of a special RNA molecule, Xist, which coats the inactive X chromosome and modifies the surrounding chromatin 34 through a gelatin-like substance. The interaction of Xist with this ‘chromosomal Jell-O’ creates a flexible environment that allows further biochemical agents to join in, leading to the silencing of gene expression on the X chromosome. Understanding these mechanisms not only sheds light on the biological foundations of gene regulation but also opens doors for potential therapies aimed at reactivating silenced genes in conditions like Fragile X and Rett Syndrome. This research highlights the importance of X chromosomal dynamics in both developmental biology and genetic medicine.
Frequently Asked Questions
What is X chromosome inactivation and why is it important for genetic disorders?
X chromosome inactivation (XCI) is a biological process where one of the two copies of the X chromosome in female cells is randomly inactivated. This ensures dosage compensation for genes on the X chromosome between sexes, as males have only one X chromosome. Understanding XCI is crucial for addressing genetic disorders linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome, because many mutations reside on this chromosome.
How does X chromosome inactivation relate to Fragile X Syndrome?
Fragile X Syndrome is an X-linked genetic disorder caused by mutations in the FMR1 gene on the X chromosome. During X chromosome inactivation, the mutated copy can be silenced, hindering its expression. Research on XCI offers potential therapeutic strategies to reactivate this gene in patients, which could alleviate symptoms associated with Fragile X Syndrome.
What potential therapies for Rett Syndrome target X chromosome inactivation?
Rett Syndrome, another X-linked genetic disorder, may benefit from therapies that aim to restore the function of mutated genes through reactivation of inactivated X chromosomes. By understanding the mechanisms of X chromosome inactivation, scientists like Jeannie Lee are developing treatments that can reactivate the healthy gene variant trapped within the inactivated chromosome, offering hope for patients.
Can X chromosome inactivation impact the treatment of other X-linked diseases?
Yes, the mechanisms of X chromosome inactivation have significant implications for treating various X-linked diseases. For instance, therapies being developed can target the inactivation process to reactivate healthy genes in conditions such as Fragile X Syndrome and potentially other genetic disorders linked to mutations on the X chromosome.
What recent advancements have been made in understanding X chromosome inactivation?
Recent research led by Jeannie Lee has unveiled the complex mechanics of X chromosome inactivation, revealing how an RNA molecule called Xist modifies the surrounding chromosomal environment. This research not only elucidates how XCI occurs but also opens avenues for developing chromosomal therapies that may help treat genetic disorders associated with the X chromosome.
How does the concept of ‘chromosomal Jell-O’ relate to X chromosome inactivation?
The term ‘chromosomal Jell-O’ refers to the gelatinous substance surrounding chromosomes that facilitates X chromosome inactivation. This substance, modified by Xist during the inactivation process, plays a critical role in rendering one X chromosome inactive and helps researchers understand the structural properties necessary for accessibility during potential therapeutic interventions.
What challenges remain in the process of X chromosome inactivation research?
While significant progress has been made in understanding X chromosome inactivation, challenges include the need to clarify why unblocking inactivated X chromosomes primarily restores the function of mutated genes while leaving other genes unaffected. Continued research is vital to enhance therapeutic approaches and develop safe clinical applications for genetic disorders related to XCI.
| Key Points | Details |
|---|---|
| X chromosome inactivation | Occurs in females where one X chromosome is silenced to prevent gene dosage issues. |
| Role of Xist | A gene on the X chromosome produces an RNA called Xist, which initiates inactivation by modifying the surrounding chromosomal structure. |
| Mechanism involving ‘Jell-O’ | Cells use a substance that functions like ‘Jell-O’ to prevent chromosome entanglement and assist in gene silencing. |
| Potential therapies | Research offers hope for therapies targeting Fragile X Syndrome and Rett Syndrome by reactivating genes on the inactivated X chromosome. |
| Future clinical trials | Plans to optimize treatments and conduct safety studies over the next few years before entering clinical trials. |
| Data insights | The research suggests a way to restore function to mutated genes while sparing healthy counterparts, minimizing side effects. |
Summary
X chromosome inactivation is a crucial biological process wherein one of the two X chromosomes in female cells is silenced to maintain gene dosage balance with males. Recent studies by Jeannie T. Lee and her laboratory illuminate the complex mechanisms behind this inactivation, highlighting the role of the RNA molecule Xist and a gelatinous substance akin to ‘Jell-O’. The insights gleaned from these studies not only clarify longstanding biological questions but also pave the way for potential therapies targeting genetic disorders linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome. As research advances, there is growing optimism regarding clinical applications that could restore gene function, presenting a significant breakthrough for individuals affected by these conditions.